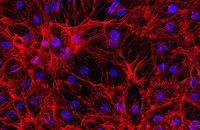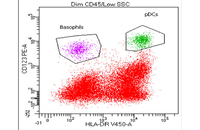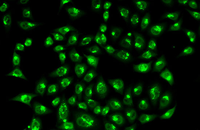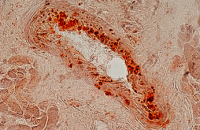
Cellular services based on the newest methods and publications
Some cellular techniques presented in Histogenotech
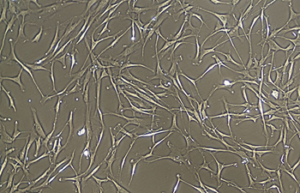
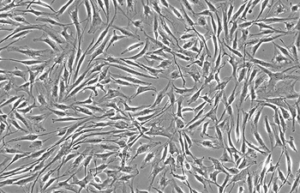
- Various types of human cells isolation and culture
- Differentiation of cultured cells into different cell lines
- Confirmation of the nature of differentiated cells by ICC method
- Evaluation of cell apoptosis by flow cytometry
- Evaluation of cell survival and proliferation
- Evaluation of cellular oxidative stress by flow cytometry
- Evaluation of cellular antioxidant by spectrofluorometric method
- Evaluation of cellular lipid peroxidation
- Investigation of mitosis cycle in dividing cells
- Investigation of cell placement on tissue scaffold
- Cell transplant from blood as IP, sub-kidney and muscle capsule
- Tracking of transplanted cells
- Cell isolation based on surface markers by MACS method
- Evaluation of cell growth, proliferation and mortality by MTT method
- Examination of living and dead cells
- Investigation of cellular contamination
- Investigation of cancer cell migration using Transwell

The use of cell culture techniques has been beneficial and crucial in genetic studies (including the transgenic animals development which can express specific genes) by inserting a foreign gene into the cell genome. In addition to cell fusion technology, which is mainly used in the production of monoclonal antibodies, as well as toxicological tests, these cells need to be cultured and maintained in the laboratory.
Cytology, by examining the structure, nutritional requirements, causes agents of growth retardation in cell growth cycle, the development of methods to control the growth of cancer cells, and the modulation of gene expression, has improved human knowledge about evolution of organisms.
Cellular study services
Various types of cells isolation and culture
The importance of performing primary culture of animal cells to evaluate the effectiveness and toxicity of new drugs, vaccines, biological drugs as well as reproductive technology has been proven. In Histogenotech, isolation of different types of cells from various human and animal tissues, including skin, fat, neurosphere, cornea, etc., can be performed using specific enzymes and mechanical methods.
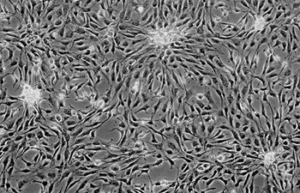
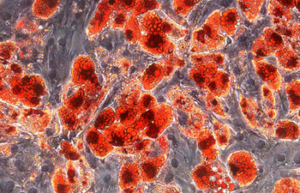
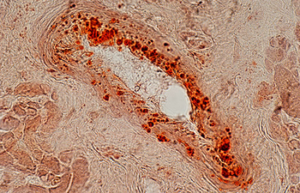
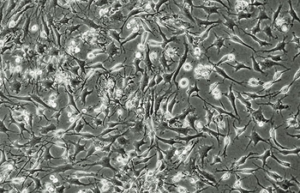
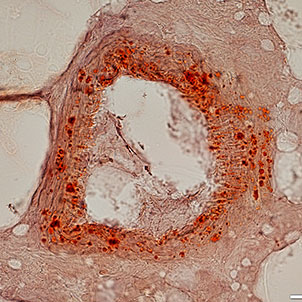
Differentiation of cells into different cell lines
Cultivation of pluripotent stem cells (PSCs) and their differentiation into different cell types has become an important factor in medicine and drug development. Histogenotech, with be beneficial of the latest technical knowledge and the best brand of cell culture media and other requirements, has been able to run the process of differentiation PSCs into different cell lines of nerve, cartilage, bone, fat, cardiomyocytes. Also, confirming of differentiation will perform using flow cytometric methods and immunocytochemistry.
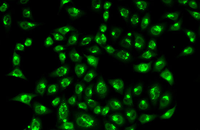
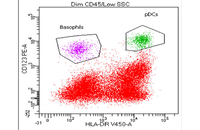
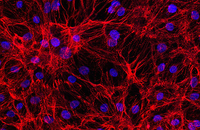
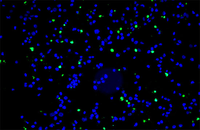
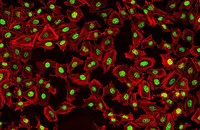
Confirmation of cell nature by flow cytometry and ICC
Demonstration of induction of stem cell differentiation into varying cell lines can be done by identifying the specific antigens of cell by immunofluorescence method. Histogenotech has been able to confirm cells differentiated into different cell types in different signaling pathways by using specific antibodies of different cell lines.
Examination of Live and Dead Cells
Acridine orange and propidium iodide (PI) are nucleic acid-binding dyes used to measure cell viability. By acridine orange staining, all nucleated cells are stained to produce a green fluorescence. While PI dye only enters cells with damaged membranes and turns dying or dead nuclear cells red. In addition, other membrane viable dyes such as ethidium bromide, 7AAD, SYTOX, DRAQ5 may be used instead of PI. In Histogenotech, cell viability is assessed by fluorescent image analysis and the ratio of live to dead cell can calculate.
Investigation of cancer cell migration using Transwell
One of the most important characteristics of malignant tumor cells is their ability to invade and migrate. Studies on cell migration are invaluable for the diagnosis, prognosis, drug development, and treatment of cancer. For this reason, there are several methods for examining this process in Histogenotech. The most common methods in Histogenotech are transwell migration assay, scratch wound assay, and molecular measurement of matrix metalloproteinase (MMP) as the major enzyme in extracellular matrix degradation.
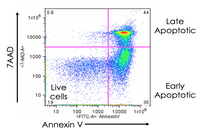
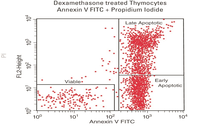
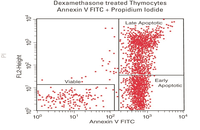
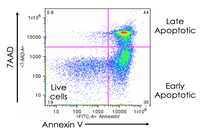
Evaluation of cell apoptosis by flow cytometry
Determining apoptosis, or programmed cell death, is important in the different animal studies and in many diseases, including cancer. At the Histogenotech Research Center, cell apoptosis is performed by a variety of methods, including cell staining with annexin PI, anxin V, tunnel and fluorescent. By histogram analysis, the results of these techniques can be reported for cell necrosis.
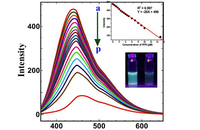
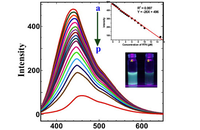
Investigation of cell antioxidants by spectrofluorometric
In Histogenotech, the level of reactive oxygen species (ROS) in frozen tissue samples can be measured based on the conversion of dichlorofluorescein acetate (DCFDA) using fluorimetry techniques. For this reason, after staining the tissue with DCFDA in the dark place, the absorbance of the samples at 488nm and 593nm is measure using a fluorimeter, which finally determines the ROS level based on the H2O2 standard curve.
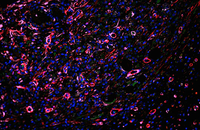
Check the placement of the cell on the scaffold
The most important part in the performance of various synthetic scaffolds is the study of cell adhesion on the scaffold. In the Histogenotech, there are various methods for examining cell adhesion that are used depending on the type and material of the scaffold. One of the most common methods of staining is cytoskeleton analysis with rhodamine / phalloidin and nucleus arrangement with DAPI. In this method, the extent of cytoskeleton and nucleus binding is assessed by fluorescent microscopy imaging.
Investigation of cellular contamination
One of the major problems of students is the presence of microbial contamination, especially Mycoplasma contamination in cell culture media. Histogenotech can detect a variety of microbial contamination by staining cells with Hoechst fluorescent dye
Evaluation of cell growth, proliferation and mortality
Evaluation of cell growth, proliferation and mortality
There are several methods for indirectly measuring cell proliferation and viability. Most of these methods measure the activity of a cellular enzyme within a living cell. One of the most well-known methods is the MTT colorimetric test. In the Histogenotech center, after examining the change of color in the environment, the number of living cells is calculated and this color change is measured using a spectrophotometer with a wavelength of 570 nm. After reading the color changes, the cell survival rate will be calculated with a spectrophotometer
Evaluation of angiogenesis
Evaluation of angiogenesis
Angiogenesis is a physiological process that leads to the formation of new blood vessels from previous ones. This process occurs naturally during fetal and organ development. However, in disorders such as inflammatory, metabolic, rheumatic, and cancerous diseases, the rate of angiogenesis is altered and excessive. In Histogenotech, angiogenesis determine using different classes of endothelial cells and matrigel and the formation of tubes resulting from cell.
Induction of cellular models
Induction of cellular models
Nowadays, using cellular studies in the treatment of common diseases such as cancer, diabetes, etc. become more important. In Histogenotech, several studies have been performed to develop cellular models and simulators to evaluate different treatments for common diseases. Induction of cellular aging using d-galactose (D-galactose) is also used in some cell lines, including astrocytes and lens epithelial cells, as a suppressive mechanism against carcinogenesis
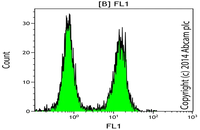
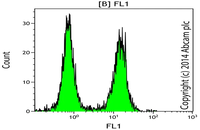
Evaluation of cellular oxidative stress by flow cytometry
Reactive oxygen species (ROS) are by-products of aerobic cell metabolism and play important physiological roles in intracellular signals. Excessive amounts of free radicals can cause oxidative damage to proteins, lipids and nucleic acids and can cause cell death. In Histogenotech, after cell isolation with trypsin EDTA and staining using DCFDA, the amount of cellular oxidative stress (ROS) is evaluated by flow cytometry.Evaluation of cellular lipid peroxidation
Malondialdehyde (MDA) is one of the most important products of peroxidation of unsaturated fatty acids in clinical specimens, which is often used to estimate oxidative stress conditions. MDA levels can be demonstrated using thiobarbituric acid reactants (TBARS). In Histogenotech using the most qualified kits, MDA is measured with TBARS in an acidic environment at 100 ° C and finally the pinkish-red product is measure using a spectrophotometer at a wavelength of 520-535 nm.Investigation of cell cycle in dividing cells
In Histogenotech, flow cytometry is used to calculate the cell dispersion in different phases of the cell cycle (G1-S-G2 / M). For this purpose, it is necessary to reveal the contents of DNA with fluorescent dye (Propidium iodide: PI). The amount of fluorescent recorded by the device is directly related to its DNA. Therefore, in different cycles of the cell cycle, as the level of DNA increases, more fluorescent signals will be recorded in the histogram.Cell transplant from blood as IP, sub-renal capsule and muscle
Cell transplantation is considered as a new clinical method for repairing damaged tissue. This transplant can be done at or away from the injury site and ultimately stimulate the repair and regeneration of damaged tissue. Stem cells have the potential for multidirectional differentiation and can differentiate into specialized cells and repair damaged tissue under certain conditions. In Histogenotech, transplantation of differentiated mesenchymal cells is used to repair and treat damaged tissue. Cell transplants can be performed at the site of injury (tumor, kidney, etc.) or away from the site of injury (intravenous, intraperitoneal, intramuscular)


Evaluation of cell survival and proliferation
Evaluation of cell survival and proliferation
In the study of cell proliferation, the results should give us a direct and accurate measurement of the number of active and dividing cells in a cell population. In Histogenotech, various methods are used to study cell proliferation. For example, the most common method of measuring the amount of DNA is using a combination of BrdU or 5-bromo-2'-deoxyuridine for cultured cells, and determining DNA amount in proliferated cells using immunocytochemistry or ELISA
Cell isolation based on surface markers
Cell isolation based on surface markers
After the initial separation of the cell from the tissue, it is necessary to separate the cell from the other cells in a specific way. In Histogenotech, in order to isolate the cells, two methods are used: FACS (separation based on fluorescence) and MACS (by nanoparticles containing iron). Existence of specific antibodies containing magnet and its columns, has provided the conditions for cell isolation and studies in the field of cell therapy at the lowest cost in this center.
Tracking of transplanted cells
Tracking of transplanted cells
In cell studies, cells need to be labeled before injection to evaluate the rate of cell migration in living tissue. In Histogenotech, there are a wide range of techniques suitable for cell tracking studies, including labeling cells with fluorescent dyes (DII, GFP, Rhodamin, etc.). To track the labeled material, a fluorescent microscope with the ability to examine different wavelengths is used.
You may find the answer to your question
Frequently Asked Questions
Due to the existence of various contaminants in the cell culture medium, which include bacteria, yeast and fungi, different diagnostic methods have been considered. In this way, to detect bacteria from deformed cultured cells, detachment of adherent cells from the bottom of the container, movement of microscopic round components (round bacteria) or rod-shaped (rod-shaped bacteria), change the color of the environment to yellow color (usually the culture medium contains phenol rejection, which is added to the culture media as an indicator of pH change and gives the culture medium a pink color) and turbidity of the cell culture medium can be used. However, in fungal and yeast contamination, the presence of budding cell clumps or yeast, and the presence of wavy fungal colonies and filaments floating on the culture medium are essential for fungal contamination.
The ATCC (American Type Culture Collection), as a biological resource center, has performed cell line authentication and quality control tests on all cell lines. Therefore, obtaining information from different conditions of a cell line using the ATCC site is a reliable way to perform laboratory tests on a cell line. Thus, when publishing cell line research, the ATCC catalog number should be included in the research materials and methods section. Accordingly, the use of cell morphology using a microscope, cell growth curve analysis and cell doubling rate, cell line detection using isozyme electrophoresis, cell DNA testing or STR analysis (DNA fingerprinting) can be used in cell line detection.
There are several methods for assessing cell survival. Tests on the permeability of vital dyes through cell membranes are the easiest way to distinguish dead cells from living ones, usually with trypan blue dye. Other colors for this tests include Evans blue color, which indicates the degree of color permeability into the tissue, indicates cell damage and death, and lack of cell staining indicates cell survival. Calcine (Calcein AM), acridine orange and ethidium bromide are among the substances that show the survival rate of cells with health and DNA damage. These can be examined with a fluorescent microscope. Compounds that show survival in response to mitochondria with the formation of formazan deposits primarily contain materials that can be examined by spectrophotometry at a specific wavelength using MTT and XTT kits. Other materials that show mitochondrial membrane potential for cell survival and health include fluorescent dyes JC1, RHODAMIN 123, and some commercial kits that are tested by flow cytometry, and finally for assessing ROS or the extent of damaged DNA the tunnel technique can use. Also, flow cytometry indicates the survival or destruction of the cells.
In order to transfer the cell from outside the laboratory to the cell culture laboratory, the cell culture medium is pre-incubated to have sufficient CO2. The cell flask is then completely filled with this medium and can be placed in an incubator-free space for one day. In this case, the unfiltered flask is closed with a full lid and transferred at a temperature below 22 ° C in a suitable container in which the culture container is fixed and away from environmental contamination. For fresh tissue transfer (Fresh Tissue), if the tissue size is less than 0.5 x 0.5 cm, it can be about 3-5 hours in cell culture medium with HPSS buffer or Hank’s Balanced Salt. Solution transferred to prevent pH changes, or transferred with physiological serum. The transfer container should be done next to the dry ice and by completely covering the lid of the container with paraffin (to prevent contamination). It should be noted that for larger cell sizes in less than 1 hour, the sample should be transfer to the laboratory.
Cell culture media can be divided into two main categories: natural environments and synthetic culture media. Natural culture media are extracted from body secretions including plasma, lymph and serum or tissue extracts. Which are classified into three categories: Biological fluids, Tissue extracts and coagulants. In this category, FBS is the most well-known product that is added to synthetic culture media. The second category is synthetic culture media that are prepared using organic and inorganic compounds. Serum-containing media, Serum-free media, chemically defined media, Protein-free media and balanced salt solutions (BSS) are classified in this group. In all of these environments there are at least a few organic, inorganic compounds, non-essential amino acids, glutamines and vitamins. The most commonly used cultivation media include:
– MEM (Minimum Essential Medium)
– DMEM (Dulbecco’s – Modified Eagle’s Medium)
– IMDM (Iscove’s Modified Dulbecco’s Medium)
– RPMI-1640 & Ham’s F-10
These mediums are different in the amount compounds and therefore have different applications for different cell lines.
Freezing is a well-known laboratory method for storing cells and other biological substances in liquid nitrogen at temperatures close to -196 ° C. For this purpose, before performing the cell freezing steps, it is necessary to first check the survival rate of cells with the vital dye trypan blue. On the other hand, the presence of Mycoplasma cell infection should be determined before cell freezing. In addition, one or two days before freezing, the cells should be passage and fresh medium should be poured on the cells to allow the cells to multiply. Cells with a density of 70 to 80% are usually frozen in a suitable culture dish and 500,000 to one million cells are poured into each cryotubes. The cell freeze medium typically contains culture medium, an antifreeze such as DMSO or glycerol, and a protein source such as FBS serum. One milliliter of freezer material is combined with one million cells and placed overnight at a freezing rate of 1 ° C / minute to -80 °C for freezing. Then, it is placed in liquid nitrogen vapor and finally in liquid nitrogen. In order to defrost the cell, the cryovial is removed from the nitrogen tank at 37 ° C to melt the contents. The preheated culture medium is then immediately added to these contents up to three times its volume, and after centrifugation at 1500 rpm, the ambient temperature is removed for 5 minutes, the previous medium and the fresh medium are added to the cell and the cell is cultured.
Cell passage is a technique that allows a person to keep cells alive under long-term culture and provide enough space for the cell to grow. In the passage technique, the cells are transferred from the old container to the new culture dishes according to the selected container and the growth rate of the cell. In this transfer, based on the rate of cell growth, the cell contents of a culture vessel are transferred to 1 to 3 new vessels. In seeding, however, the cells are separated from a single container and transferred to a certain number for a specific laboratory test in the same container or culture vessels with more or less dimensions. Therefore, in this case, the goal is not cell proliferation and only control of the cell population at the beginning of a test or selection of a culture dish to perform a specific test is considered.
Drug screening in biomaterials can be useful for better prognosis in the early stages of preclinical drug production. Drug response assays assess the effect of a drug on cell populations over a range of concentrations. Therefore, in order to achieve pharmacological concepts such as IC50 (drug inhibitory concentration at which the cell population is reduced by half), EC50 (effective drug concentration at which the maximum cell response concentration is obtained) and Emax (maximum drug effect), The MTT method is used to evaluate the results of drug response measurement and to characterize drug potency. However, in order to obtain the concentration range of a biological substance or drug, comparing drug responses in different substrates and conditions in published articles can be challenging. Thus, the results of applications and drug form of a substance in drug response criteria and differences in cell growth rates for cells cultured in different biomaterials are different in studies. Therefore, using this research, the concentration range of a substance is determined and this range is evaluated by MTT test.
Drug efficiency for inducing cancer cell mortality can be evaluated by various laboratory tests. In this regard, the effect of drugs on preventing cell growth can be investigated by MTT method. Induction of cell death or apoptosis can be evaluate using flow cytometry with Annexin-PI kit and tunnel technique. On the other hand, stopping the process of differential cell mitosis can be performed using flow cytometry and cell cycle analysis. In order to evaluate the extent of cell invasion or migration, to evaluate the extent of cancer cell metastasis by cell scraping method or cell migration is done through the pores of trans-vessel vessels. The effectiveness of cancer drugs in inhibiting angiogenesis can be done through tube formation test and finally the use of gene markers or protein mechanism of action of anti-cancer drugs that can be done by molecular tests such as PCR or western blot and immunohistochemistry.
The different effects of a drug in terms of increasing proliferative power or increasing cellular resistance to environmental stress can be done with different tests. In this regard, in addition to the effectiveness of a plant extract with MTT test, in order to determine the effectiveness of a plant extract using its antioxidant properties, ELISA test or antioxidant enzymes such as SOD, GPX, CAT, GSH, TAC are used. Also, to evaluate the amount of environmental stress that the plant extract is able to reduce or eliminate these effects, we can refer to the ROS test by flow cytometry or MDA test by spectrophotometer. The properties of the plant can be studied by GC or HPLC in terms of examining its various components
Labeling and tracking cells are important processes in understanding the biological mechanisms and therapeutic effect of inoculated cells in vivo. There are many ways to label and track inoculated cells in vivo or in the laboratory. Staining the cell with DiI fluorescent is one of the most common methods of cell marking. By binding to membrane phospholipids and other membrane organelles, this pigment is able to attach to new cells by dividing cells and show the proliferation of inoculated cells in the body. On the other hand, the use of GFP protein, which is done by manipulating the cell genome and transferring the GFP gene to the host genome, is the second most common method in marking cells. In this method, the process of cell labeling is more time consuming than the previous method, but due to the transfer of the labeled substance to the cell genome, the color stability in several generations of amplified cells is higher and the rate of cell proliferation is more accurate. Other materials such as Brdu and Hoechst vital dye are also used to mark the cell. These materials also bind to cell DNA and show the amount of cell proliferation in the body. The latter two methods are superior to the previous two methods for some cell studies, including proliferation and mitosis of transplanted cells, and are not used solely for cell tracing.
Cell counting in a cell culture laboratory is one of the essential and common methods at the beginning of any laboratory test. Knowing the initial cell population at the beginning of the test and comparing this population at the end of the test provides the researcher with detailed information on how effective the treatments are and the diagnosis of some diseases. In this regard, using a hemocytometer slide (neobar slide), the cells isolated from the bottom of the container after centrifugation were homogenized in a volume of one ml of culture medium and then 10 μl of the cell with 10 μl of trypan blue dye in The sterile conditions are mixed together to place a 10 μl drop from the cell on a hemocytometer slide. The enumerated cell population in large cells was counted on a hemocytometer slide and after obtaining the average cell population in the enumerated cells taking into account dilution factor 2 (due to combination with trypan blue) and the initial volume in which the cell was diluted, Cell population is presented in units of volume.