Bacitracin Susceptibility test
Bacitracin Susceptibility test
Purpose
This test is used for presumptive identification and differentiation of beta-hemolytic group A streptococci (Streptococcus pyogenes- susceptible) from other beta-hemolytic streptococci. It is also used to distinguish staphylococci species (resistant) from micrococci (susceptible).
Principle
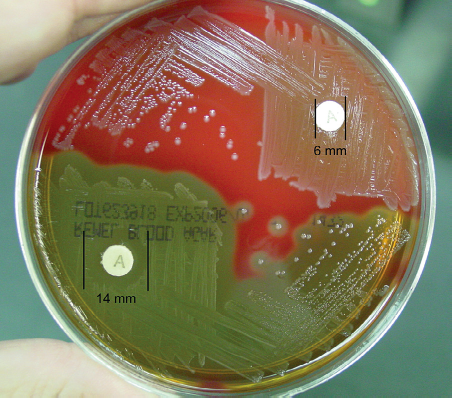
The antibiotic bacitracin inhibits the synthesis of bacterial cell walls. A disk (TaxoA) impregnated with a small amount of bacitracin (0.04 units) is placed on an agar plate, allowing the antibiotic to diffuse into the medium and inhibit the growth of susceptible organisms. After incubation, the inoculated plates are examined for zones of inhibition surrounding the disks.
Method
1.Using an inoculating loop, streak two or three suspect colonies of a pure culture onto a blood agar plate.
2.Using heated forceps, place a bacitracin disk in the first quadrant (area of heaviest growth). Gently tap the disk to ensure adequate contact with the agar surface.
3.Incubate the plate for 18 to 24 hours at 35°C to 37°C in ambient air for staphylococci and in 5% to 10% carbon dioxide (CO) for streptococci differentiation.
4.Look for a zone of inhibition around the disk.
Expected Results
Positive: Any zone of inhibition greater than 10 mm; susceptible
Negative: No zone of inhibition; resistant
Limitations
Performance depends on the integrity of the disk. Proper storage is positive (Streptococcus pyogenes); growth up to the disk is negative and expiration dates should be maintained.
Quality Control
Positive: Streptococcus pyogenes (ATCC19615)-susceptible Micrococcus luteus (ATCC10240)-susceptible
Negative: Streptococcus agalactiae (ATCC27956)-resistant Staphylococcus aureus (ATCC25923)-resistant
REFERENCE:
Bailey & scotts
Diagnostical microbiology
latest edition
Related Content: Acetate Utilization test – drug-carrier nanoparticles
