Molecular genetics and cytogenetics services
Some of the techniques presented in Histogenotech
- Perform ELISA, Western blot and flow cytometry
- Bacterial Transformation by chemical method and electroporation
- Bacterial colony culture, plasmid purification and virus generation from vector
- Perform PCR cloning on expression vectors
- Design, order and check the accuracy of vector construction
- Design expressive vectors
- Cell transduction with virus
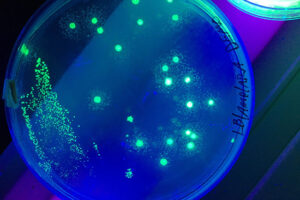
- Labeling the cell with GFP vector
- Production of recombinant cell line
- Educational cloning of genes for protein expression
- SDS PAGE technique
- Kumasi Blue staining

Today, due to the increasing concerns of genetics studies, especially in Iran, the application of various genetic tests in the fields of stem cell research, diagnostics, treatment, prognosis and many other areas become very important. Histogenotech research laboratory intends to introduce the processes, applications, disadvantages and advantages of some of the most widely used genetic tests in both cytogenetics and molecular genetics after briefly introducing various types of genetic tests based on their functional purpose.
Therefore, in the field of cytogenetics, genetic manipulation tests, flow cytometry, FISH, CGH and in the field of molecular genetics, various methods based on PCR, PCR quantification and new generation sequencing techniques (NGS) are introduced.

Molecular Genetics Studies Services
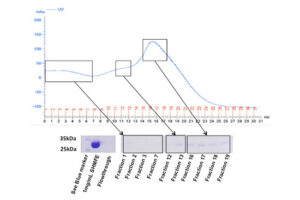
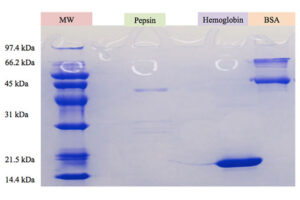
SDS PAGE technique
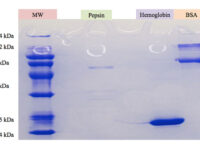
The SDS-PAGE technique is a fast, reproducible and low-cost method for proteins studies. This method is commonly used to investigate the purification steps, calculate the relative amount, and determine the molecular weight of proteins. This technique uses sodium dodecyl sulfate (SDS), which binds to proteins to cover their natural charge and creates a uniform distribution of negative charges on it, which binds to the hydrophobic regions of proteins and denatures them. Since many industrial projects are being carried out in Histogenotech Company and in each research project accuracy of study should confirm, it is necessary to carefully examine the proteins. This technique is able to accurately separate proteins based on their molecular weight. Polyacrylamide gel has very effective role in the separation of proteins in SDS-PAGE. The pore diameter of the polyacrylamide gel determines the separable weight range in SDS-PAGE. In this way, with different concentrations of gel, various proteins can be separated from each other.
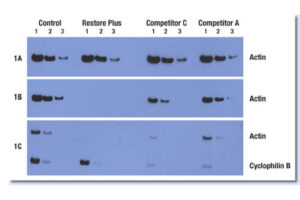


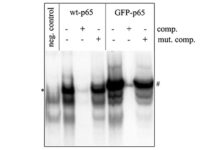
Western blotting technique
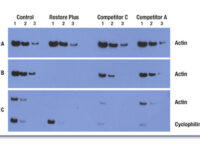
Western blot is a laboratory method for distinguishing specific protein molecules from a mixture of proteins that is widely used in cell-molecular biology and immunology. This protein mixture can contain all the proteins associated with a particular tissue or cell. Numerous preclinical studies can be performed in the protein analysis laboratory of Histogenotech Company due to the existence of a diverse antibody storage that corresponds to different species of animals, including mice, rats, rabbits and humans. In these studies, the size of the desired protein can be evaluated using the Western blotting technique and the amount of protein expression can be measured. Western blotting is widely used in biochemical studies to qualitatively identify individual proteins and modify proteins (such as post-translational changes). This technique is commonly used to confirm protein production after cloning as well as in medical diagnosis.

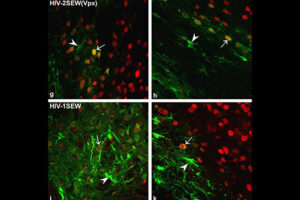
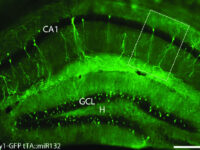
Labeling the cell with GFP vector
One of the most novel methods for investigating the expression of recombinant proteins is the use of reporter genes in vector design. These vectors can be used to track the expression of the gene in cell, bacterial or yeast culture. Vectors that carry the green fluorescent protein (GFP) tracer gene are often used as reporter genes to report the expression of genes cloned in the vector or to track stem cells in vivo. In the biotechnology laboratory of Histogenotech, using lipofectamine, eukaryotic cells of different normal and cancerous categories were transduced with vectors containing GFP in doctoral and master’s thesis in different fields of basic medical or interdisciplinary sciences and after examination at different times. After infecting the cells with the virus, the expression level of GFP and the number of positive expression cells, counting and the efficiency of transfection and transduction methods are evaluated. GFP protein expression in transgenic animals can also be assessed in subsequent generations of modeled animal cells.
Perform PCR cloning on expressive vectors
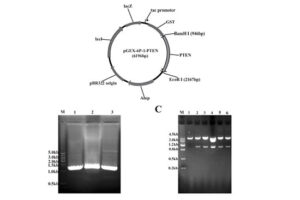
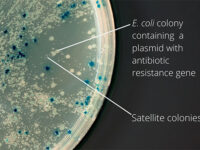
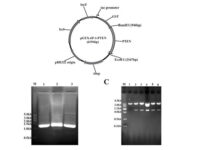
After plasmid extraction, clonal PCR method is used to confirm the presence of the gene in the plasmid. Colony PCR is a technique used to examine the presence of DNA in plasmids inserted into bacteria and yeasts by the transformation reaction. The PCR cloning technique is performed directly on bacterial or yeast colonies, during which the target sequences are amplified directly by PCR without the need for DNA extraction and purification prior to PCR. Experienced specialized staffs in the molecular department of Histogenotech Company, in order to advance research projects in support of student projects, provide free consulting services to design suitable primers for PCR colonization. The primers are designed and synthesized before PCR cloning, and finally the PCR products are electrophoresed on agarose gel to observe the desired band. If the gene is in the right place, observing the band on the gel electrophoresis indicates that the colon selection process in the medium containing the appropriate antibiotic has been performed correctly.
Cell transduction with virus
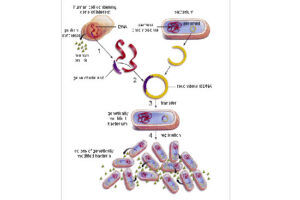
Transduction is the process by which bacterial DNA is transmitted from one bacterium to another through a virus (bacteriophage). Generalized transduction is performed by lethal phages, and bacteriophage carries only bacterial DNA. So in this case the recipient cell will survive. After phage infiltrate the host bacterium, fragments of bacterial DNA remain intact and are randomly inserted into the phage capsid head. Phage will inject a bacterial DNA fragment it carries randomly into another bacterium. If there is a homology between the newly injected strand and the bacterial genome of the receptor, the fragment may be attached to it. The gene on that fragment can encode a protein that the receptor did not initially have, such as a protein that inactivates an antibiotic. The transferred gene fragment is protected from destruction by translocation by the phage capsid that holds it in place. Specialized transduction occurs by moderate phages (lambda phages). When the virus is activated, there is sometimes an error in cutting phage DNA, and a piece of bacterial DNA on one side of the phage is cut, cloned, and packaged with phage DNA. This action can lead to the transfer of that piece of bacterial DNA to other bacteria, and eventually it can give the bacterium a new capability.
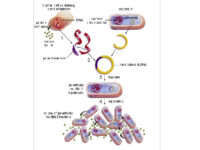
Bacterial colony culture, plasmid purification, virus generation from vector
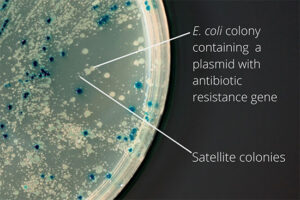
After the transformation, the transformed bacteria are cultured in liquid medium. Biomarkers are used to select bacterial cells that have received the plasmid. Biomarkers are usually a fluorescent protein or an antibiotic resistance gene. In order to evaluate the ability to produce a specific protein from a cell in clinical and preclinical studies, the use of expression vectors is very valuable. Plasmid purification in biotechnology laboratory Histogenotech is provide by selecting the bacteria carrying the plasmid, which is provide by culturing the bacteria in a solid culture medium containing a selective marker (antibiotic). In the molecular unit of Histogenotech biotechnology laboratory, plasmid purification is performed with a special commercial kit according to the manufacturer’s instructions. Methods for confirmation of recombinant plasmid in transformed bacteria include enzymatic digestion using restriction enzymes, sequencing and PCR colony using specific primers. Finally, for the initial confirmation of the presence of the desired gene in the plasmid, enzymatic cleavage is performed using the restriction enzymes and the presence of the expected fragments on the agarose gel will be confirmed by electrophoresis. Due to equipped microbial biotechnology laboratory of Histogenotech with TOP10 and DH5 E. coli host strains and designing and manufacturing bacterial-yeast expression vectors as well as plasmid extraction kits, biotechnology projects can be performed with the highest quality.
Bacterial Transformation by chemical method and electroporation
Transformation is a key step in the DNA cloning process. Different vectors are used depending on our purpose of the transformation as well as the size of the part to be transformed. Bacteria normally cannot receive extra-genomic DNA on their own. In general, two chemical and electrical methods are used to transfer the vector carrying the genome into the bacterium. The first method is to transfer the vector chemically. In this method, which is performed in microbial laboratory of Histogenotech, calcium chloride is used to create competitive bacteria. In this way, the bacteria are first placed in a cold solution containing calcium ions, which leads to the neutralization of the electrostatic repulsion between the negative charges of the membrane phospholipids and the negative charges of the vector DNA, and finally facilitates the process of vector transfer to the cell. In the second method, an electric field is used to increase the permeability of the bacterial wall (electroporation), which is done in Histogenotech Company.
Coomassie blue staining
Coomassie blue staining is a family of dyes commonly used to stain proteins in SDS-PAGE gels. To detect proteins after electrophoresis, SDS-PAGE gels are soaked in dye and then the excess dye is removed with a solvent (dye). This method allows the proteins to appear as blue stripes on a bright background. In most projects, protein expression is examined by Western blotting to determine the concentration of a protein and also the presence or absence of a specific enzyme or hormone in various biological samples. In our projects, in order to produce biotechnology products or drug design, the use of Coomassie Blue dye to stain the proteins can show the result of activity to the researcher in the fastest time. Histogenotech has several projects in its protein laboratory using this dye in protein testing. Coomassie Blue staining uses ammonium sulfate solution, Coomassie blue solution, orthophosphoric acid solution and ethanol. In less than two hours, this method stains the proteins on a clean background.Production of recombinant cell line
Advances in recombinant DNA technology have expanded our knowledge of the molecular mechanisms of virus replication. Virus replication in cells occurs as a result of complex interactions between virus-associated agents, virus-encoded substances, and host cell. These complex interactions require expertise in various fields in order to achieve the highest efficiency in the function of the manipulated cell. In the Molecular and Virology Laboratory of Histogenotech, specialists in the fields of biotechnology, bacteriology, microbiology, biochemistry, and cell-molecule in a coordinated interaction have succeeded in producing different classes of eukaryotic transgenic cells that can be used to study the inductive effects of protein. Due to the key role of secretory proteins from manipulated cells in the production of recombinant drugs and monoclonal antibodies, it has been considered by many researchers and pharmaceutical companies active in this field in many clinical and pre-clinical studies. These recombinant cells can be stored in a cell bank for therapeutic, diagnostic, or production purposes.





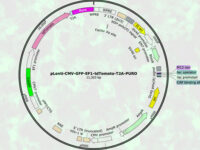
Design expressive vectors
Most recombinant proteins can produce by cloning genes in gene vectors or vectors. In the molecular unit of Histogenotech, expressive vectors with various gene structures are designed to achieve optimal levels of recombinant protein expression. In the high-technology industrial studies using transgenic or manipulated cells, increasing production speed and reducing costs can affect the success of the product, which is achieved by designing a vector with precise details. Thus, during various research projects, by providing high-tech laboratory equipment, by maximizing the efficiency of the recombinant protein production process, the performance of the produced protein and the effectiveness of protein binding to its receptor in the target tissue can be affected. Design of vectors based on lentivirus, retrovirus, adenovirus and various other transgenic products is provide in biotechnology laboratory of HistogenotechDesign, order and check the accuracy of vector construction
Because the design of expressive vectors (yeast and bacterial) requires bioinformatics specialists, cellular and molecular laboratory of Histogenotech with bioinformatics services and computer analysis, is able to design a specific vector structure or function vector carrying gene fragments to predict. Knowledge of designing a suitable primer to track the target gene fragment in a vector requires a high level of experience in the molecular cell field. In addition, the production of oligonucleosides required in the design of primers in the process of verifying the function of the gene fragment in the vector, in the amount of secreted protein and its effectiveness, can provide a great help in the production of technological products of researchers.Perform ELISA technique
ELISA (Enzyme-linked immunosorbent assay) is one of the most widely used immunoassay techniques used to detect an antigen in cell secretions, serum or blood plasma. In this technique, the detector antibody binds to the enzyme. The presence of an antibody or antigen and an enzyme involved in this process leads to the production of a signal. In order to test for antigens in a sample, it requires detection antibodies, which are available in the hormone and biochemical laboratory of Histogenotech Company. Different types of polyclonal and monoclonal antibodies are available to study different species of mice, rats or rats, rabbits and humans. This diversity of antibodies in the Histogenotech antibody bank has made it possible to study serum, plasma or cell lysis extract in various research projects, in the studies of professors, or master’s and PhD students. Most studies use serum or plasma samples from laboratory animals, which are designed depending on the purpose and type of analyte being measured in different types of ELISA, including: direct, indirect, sandwich and competitive ELISA.Perform flow cytometry technique
Flow cytometry is an accurate, high-performance method for identifying cells (particles) and evaluating their properties. Each cell expresses its own molecule on the surface or inside the cytoplasm, depending on the type and specialty it is responsible for. In this way, the cells express a specific marker on their surface according to the task they perform during differentiation and according to the environment in which they are located, which can be identified by flow cytometry technique. In the molecular laboratory of Histogenotech, with the beneficial of a diverse antibody bank, it is possible to detect different types of normal, cancerous, differentiated, manipulated (transgenic) cell lines or to detect cellular proteins by flow cytometry technique. The study of cell-specific, intracytoplasmic or nucleus-specific proteins in student dissertations at different levels of master’s and PhD studies is one of the concerns of students and therefore the existence of a rich bank of antibodies can facilitate the implementation of various research projects. Fluorescent dye-linked primary and secondary antibodies can detect and attach to surface molecules or internal compounds in cells, making it possible to identify cell types in a diverse cell population.

You may find the answer to your question
Frequently Asked Questions by Customers
The non-operation of the vector can be detected in several ways. The vector transformed in the bacterium must have a selective marker of antibiotic resistance. In this way, if it does not act, it cannot grow in the environment containing antibiotics. The vector must have a reporter gene so that it can be seen under a microscope after the target gene is actuated in the eukaryote. In case of incorrect design or not considering the correct enzymatic cutting sites in the vector, or selecting the inappropriate vector suitable for the structure or selecting the wrong host to secrete the protein, the vector will not work and each case must be re-evaluated.
Expression vectors are plasmids used to express the desired gene in a cellular host. Choosing the right expression vector is the key to the success of research product development and biotechnology projects. Thus, depending on the type of protein and the prokaryotic and eukaryotic host used, the sequence design of the structure in the vector under different promoters can affect the amount of protein produced. In addition, basic vector types including lentiviruses, retroviruses, adenoviruses, and other types of basic vectors can affect the efficiency of the technique.
The lac promoter is one of the most common promoters used to express heterologous proteins in E. coli. Isopropyl-β-D-thiogalactoside (IPTG) is currently the most efficient molecular inducer to regulate the transcriptional activity of this promoter. IPTG can increase the amount of protein secreted in prokaryotes. This compound is similar to alolactose as a lactose metabolite that stimulates transcription of the lac operon and is therefore used to induce E. coli protein expression where the gene is controlled by the lac operator.
In order to manipulate the genome of a eukaryotic and prokaryotic cell, there are several steps that must be performed accurately and sequentially. After placing the structure in a viral vector, to increase the concentration, the vector is transformed into bacteria. This step is chemical or electrical, and after transforming and culturing the bacteria, the positive clones are isolated and finally the positive bacteria multiply and purify the plasmid, and by transduction in the eukaryotic cell, leads to a change in the genome. In the transformation stage, PCR colony tests to check the presence of vector in bacteria, PCR test to confirm the presence of the desired fragment in the vector, after transduction, observation of GFP as gene reporter with fluorescent microscope, to evaluate the amount of secreted protein from ELISA and Western test. Blotting and performance testing are used in vitro and in vivo.
The bacterium is intended as a host for the purpose of vector replication or for use in the secretion of target proteins. Bacteria are naturally sensitive to many antibiotics. Therefore, to select a bacterium that contains the target gene in the vector design, an antibiotic resistance gene called a selective marker is inserted. This gene, when present in the bacterium, leads to the survival of the bacterium in culture conditions containing antibiotics and the bacterium can multiply. In this way, it is separated from other bacteria and forms a colony, which can confirm the presence of the desired fragment in the bacterium by Colony PCR method.
In order to evaluate the secretion of protein in a manipulated cell, the function of the gene can be estimated by first observing the expression of the Reporter gene, which is generally GFP. On the other hand, the expressed gene and the resulting secretory protein are evaluated for ELISA techniques in terms of concentration and SDS-Page and Western blotting for molecular weight and purity. In addition, the protein activates some intracellular molecules and stimulates some, using molecular techniques such as PCR, using effects from the function of gene cascade pathways.
There are several considerations in designing a vector, including the correct choice of the base vector based on the final host. In this way, the sequence of the structure from the NCBI site is initially viewed as a genomic library and the part involved in protein construction from the beginning to the end codon is considered along with the restriction enzyme cleavage sites (Restriction Enzyme). The sequence is then ordered to be constructed and placed in a circular vector.
Green Fluorescent protein (GFP) is used as a reporting gene during the secretion of a protein in a host cell, or as a cell tracker in in vivo studies. If the gene for this protein is permanently inserted into the cell genome under a promoter designed in an expression vector, the gene will be inherited each time the cell divides and will be permanently visible. However, if the vector is transiently inserted into the genome, it will be active for a limited time, and eventually the GFP will be removed by removing the vector function.